 |
|
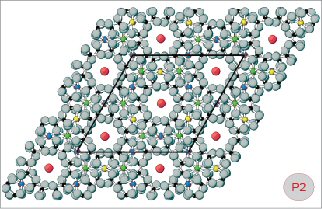 |
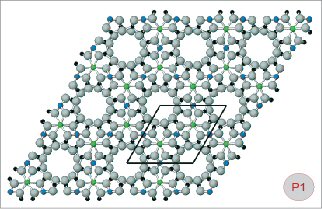
Fig. P1-2
Ball-and-stick models of beryl (Fig. P1) and pezzottaite
(Fig. P2). A projection of the crystal structure in the
direction of the c-axis is shown in order to explain some
details of the ring systems in these two minerals. The size
and position of the unit-cell (as seen perpendicular to
the c-axis) is also shown. Green balls
= aluminium cations (Al), blue
= beryllium (Be), yellow =
lithium (Li), red = caesium
(Cs), grey = oxygen (O),
black = silicon (Si). The structure of beryl is characterized
by six-membered rings of SiO4 tetrahedra alternating along
the c-axis with twelve-membered rings composed of alternating
BeO4 and AlO6 polyhedra. In the mineral pezzottaite, an
arrangement of beryl-like rings is found, but there are
two different types of twelve-membered rings: (1) Those
identical to the corresponding rings in beryl, and (2) those
assembled of LiO4, BeO4 and AlO6 polyhedra. To allow for
the ordered (Be, Li) distribution in the structure of pezzottaite,
the unit-cell had to be enlarged relative to beryl (compare
P1 and P2). Both models are represented in hexagonal settings
for better comparison. It must be remembered, however, that
pezzottaite has a rhombohedral crystal lattice and beryl
has a hexagonal lattice. In trigonal/rhombohedral symmetry,
the highest symmetry element is a three-fold rotation axis,
whereas in hexagonal symmetry a six-fold rotation axis is
required. |