| |
 |
| |
|
| |
Sometimes
it is absolutely amazing that an existing technology that could
answer a particularly important need is completely overlooked
as an entire industry looks everywhere else instead of at other
industries already making use of what they need in the first
place. This is the case with the detection of the presence of
beryllium in corundum. When the beryllium diffused controversy
first surfaced, the international laboratories were looking
at analytical methods which would give them quantitative chemical
analyses in order that they could determine with greater accuracy
what elements were involved and how they interacted with the
existing chemistry of the stones to create the colours that
were resulting from this new heating technique. The detection
system of choice at the time was the Laser Ablation Inductively
Coupled Mass Spectrometry (LA-ICP-MS) and the Secondary Ion
Mass Spectrometry (SIMS).
However
these systems did not provide that best solution for routine
gem testing. The problem with these two systems is that they
are so sophisticated and expensive that less than a handful
of laboratories worldwide have access to this machinery. Not
only is the machinery extremely expensive to buy, it is also
expensive to maintain and the training costs of the operator
and the cost of operation is similarly very high. This factor
alone makes it unviable for detection of beryllium (Be2+) in
all but the most expensive and rare stones. |
|
|
| |
Thus
it was that the Gemological Institute of America (GIA) decided
to look elsewhere for an alternative to LA-ICP-MS and SIMS.
The solution was not very far away from a technology standpoint.
Laser Induced Breakdown Spectroscopy (LIBS) was being used
for analytical and detection purposes in other industries and
has a number of applications. |
|
Mr. Christtopher Smith operating LIBS at the 34th
Bangkok Gems & Jewelry Fair. |
| |
A team of researchers
from the GIA began investigating if the LIBS analytical technique
could be modified to suit the needs of gemmological research and
offer a viable option for routine testing. |
| |
 |
|
Before
we go on to talk about what the GIA is doing with LIBS,
it is necessary to first understand what it is. Laser
Induced Breakdown Spectroscopy or LIBS is an analytical
detection technique and sensor technology that
is undergoing a dramatic transformation in terms of
hardware, software and application areas. It is a field
that is significantly maturing and also expanding into
dramatic new areas. The core of the technique is laser
spark spectrometry or the spectral analysis of laser
induced sparks (also known as optical emission Spectroscopy).
In the
late 1990's to the beginning of the 21st
century, the field of LIBS has significantly developed
in the area of component instrumentation, especially
in the development of broadband high resolution spectrometers
which allowed for the detection of almost all the chemical
elements in the periodic table and beryllium is one
of them.
LIBS
allows for the rapid on site analysis of solids, liquids
or gases. The way it works is that a powerful laser
beam is focussed on a sample material and some of the
matter in the sample is vapourised and a hot spark (plasma)
is formed. The light emitted by the plasma is composed
of specific emission lines that are characteristic of
the elements present in the sample. By analysing the
spectral emission of this light, it is possible to deduce
the elemental composition of the material under study.
For gemmology, this technique shows the greatest application
as a highly sensitive, qualitative analytical technique.
However, dependant upon the substance being examined
and the elements in question, under certain circumstances
it may be possible to corelate a proportion of spectral
line intensity to elemental concentration, thus allowing
for a semi-quantitative analysis. |
| The inner chamber of the LIBS machine. |
|
|
| |
|
|
|
|
| |
This
technique allows for the analysis of materials with virtually
no sample preparation, on site, real time (rapid) analysis,
stand off analysis (only optical access is required), it is
field portable and very high resolution. Its applications vary
from the fields of dentistry to space technology, from analysing
the levels of lead in water to detecting the presence of chemical
warfare agents. |
| |
GIA
has adopted this technology and developed a prototype
instrument together with Ocean Optics Inc. that is focussed
on gemmological applications and the detection of beryllium
in particular. Christopher Smith, Manager of Gemmological
Research, GIA GTL. was recently in Thailand during the
34th Bangkok Gems & Jewelry Fair, where he presented
the instrument to the local local industry at the GIA
GemFest. Unlike, LA-ICP-MS and SIMS, this does require
a lot of space or highly trained professionals. In fact.
Christopher was able to train the GIA Thailand staff to
use the machine in a matter of minutes.
Presenting
this in Thailand was particularly important as the adaptation
of this technology came about as a result of one of the
greatest challenges the gemstone industry has faced in
recent years. The search for a viable detection process
for the identification of beryllium in corundum. It had
become very important to create a routine testing system
for beryllium identification. In fact, there is a lot
about beryllium diffusion that is not understood yet.
Christopher says that when he spoke to the Thai traders
at the fair, many were not aware of the full spectrum
of colour that may result when rubies and sapphires are
heated with beryllium (Be). Most traders thought that
Be diffusion was mainly limited to various shades of orange
and yellow.
One
of the most attractive features of LIBS is its low cost
that makes it possible to analyse even commercial goods.
Christopher indicated that the ease of operation which
this unit offers makes testing single stones straightforward,
although further development of the system will involve
modifications to the sample stage that will more readily
facilitate the testing of gemstone "lots". One
will then be able to take parcels of rough or polished
goods and very quickly analyse each and every piece in
the parcel to separate those that were heated with beryllium
and those that were not.
|
|
|
| |
The LIBS hardware does not take up much more
space than a desktop computer. |
The
low cost of the machine will also enable every gem
lab to afford one, as it does not cost much more
than a standard UV/Vis/NIR and IR spectrometer used
by most gemmological labs. In fact, even major suppliers
of gemstones will have the ability to purchase a
LIBS unit and keep it in house for bulk testing
of goods. As mentioned earlier, it is easy to operate
and once the alignment is set up, even a novice
can run the machine. It does not have a high maintenance
cost either. In fact, apart from the cost of the
machine itself and the |
 |
|
argon
gas, which is used in the sample chamber, there
is no other investment required. Christopher indicated
that the GIA is very optimistic about the application
of this LIBS unit. The prototype is specifically
geared towards the detection of beryllium. Initial
testing has positively identified the presence of
Be in very low concentrations of a few parts per
million. Other units are also planned that will
have an expanded analytical capability allowing
for the simultaneous analysis of up to 55 elements.
As
of now, GIA has only the one unit, Christopher says
that they are still developing it and plan to refine
the instrument further. In fact, he says with a
smile, the trade in Bangkok has seen the unit before
most of the GIA staff in New York and the Institute's
Carlsbad headquarters even got a peek at it. Well,
it is quite fitting that the machine makes its debut
here in Thailand as Bangkok is after all the gemstone
capital of the world, and so, might just turn out
to be its largest market.
|
| The sample chamber inside
the machine. |
|
|
|
|
Abstract
paper presented by Ted Themelis at the International
Gemmological
Conference in Wuhan,
(China) on September 12-18, 2004 |
| |
|
|
This
work presents a preliminary study dealing with the
detection of elements in gems using the Laser Induced
Breakdown Spectroscopy (LIBS) method. The purpose
of this project was to investigate the overall performance
of the LIBS method for detecting beryllium (Be),
lithium (Li) and boron (B) in untreated and treated
rubies-sapphires, as well as chrysoberyls and beryls.
Most of these corundums were heated by the author
with/without beryllium, lithium in his thermo-chemical
lab in Bangkok.
The
elements analysed in these specimens were performed
with a bench-type LIBS configuration setup. Laser
sparks fired into the surface of the specimens by
focused pulses from a laser produced plasma with
distinct spectral emission signatures which were
captured by a spectrometer and processed by a computer.
All observations and testing procedures were recorded.
The ionic emission lines of Be, Li and B as well
as the atomic lines of Al were detected and numerous
spectrograms were produced. Detection limits of
Be, Li and B in corundum, damage assessment of the
specimens, |
|
 |
| |
|
Ted Themelis working with
LIBS in the U.K. |
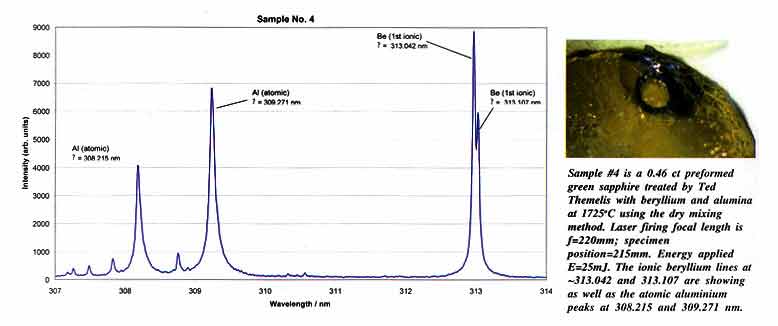 |
| The
results of this study showed that the LBS method can
detect Be, Li. B and other elements in corundum. Based
on the number and quality of the specimens tested
the following observations were made: |
1. Qualitative measurements
tolerance of error was about 20%.
2. Quantitative measurements could not be achieved.
3. Detection limits of beryllium in corundum was determined
about 3 to 4 ppm.
4. Calibration curve for beryllium in corundum could
not be produced.
5. Calibration using master samples Be-treated and
Li-treated corundums is possible, but limited.
6. The LIBS method was termed "slightly destructive"
for gemological testing.
|
|
In
conclusion, the LIBS method used as a tool for detection
of Be, Li, B and other elements in corundum and
other gemological investigations is strongly
indicative, but not conclusive.
The current generation of the LIBS instrumentation
will not replace the well-established LA-ICP-MS
and SIMS methods.
It
is emphasised that this study is a preliminary account
of the applications of LIBS in gemology and verification
of the facts uncovered in this investigation must
be substantiated with future experiments and research.
|
|
|
|
| |
 |
Topaz
is actually a fairly common gemstone and is worn
around the world in its blue avatar. However, naturally
occurring blue topaz is extremely rare and the many
varieties of blue topaz set in inexpensive jewellery
around the world is actually irradiated material.
Until the 1950s though, topaz was generally known
as a yellow or golden coloured gemstone.
Topaz
has been used in jewellery for centuries. The name
itself is said to derive from the Sanskrit word
Tapas meaning fire or from the island of Topazios
(Zabargad) where peridot was found. It is believed
to be one of the gemstones on the breastplate of
Aaron. The ancient Egyptians
associated its colour with the golden glow of their
mighty Sun God Ra.
This made topaz a very powerful
amulet in their estimation and they believed that
it protected the faithful from harm. The ancient
Romans too associated the gem with their Sun God
Jupiter. |
 |
1. Irradiated blue
topaz
|
|
|
There
are many legends associated with topaz. The Greeks
believed that it had the power to increase strength
and even make its wearer invisible in times of danger.
It was also believed that the stone would change
colour in the presence of poisoned food and drink,
a very important quality to possess in ancient times.
It was said to dispel enchantment and improve eyesight
Its mystical and curative powers were believed to
wax and wane with the phases of the moon. Ground
topaz mixed with wine was used in the treatment
of asthma, insomnia and haemorrhages.
|
|
|
| |
|
 |
|
Topaz
was a popular talisman with the Europeans of the Middle
Ages who believed that it protected the wearer from having
bad dreams, calmed passions and ensured faithfulness.
There is a story that Lady Hildegarde, wife of Theodoric.
the Count of Holland, presented a topaz to a monastery
in her native town. The gem emitted such a bright light
at night that prayers could be read without the aid of
candles in the chapel where it was kept. Of course, one
would expect that the monks would already have known the
prayers by heart so the story can be dismissed as the
stuff of legends. It serves to point to the past popularity
of this gem though which is in stark opposition to its
current position as a low-end gemstone. In fact in the
19th century when pink topaz was discovered in the Ural
Mountains, the Czar of Russia restricted the gems from
those mines for the exclusive use of the Imperial family
and its selected favourites. Thus the gem got its name
as Imperial Topa/. Today the term imperial is reserved
for pink, orange and red topaz. |
|
Spice Topaz is a brand name of
diffused topaz created by India's Pink City Gem Technologies.
|
|
Topaz
commonly occurs in colourless or brown. Rare varieties
include golden, pink, red, purple and green. There is
some naturally occurring blue topaz but it is extremely
rare. These natural coloured topazes can be classified
as fine gems. The colours of some natural topaz, most
notably Siberian topaz, can change when exposed to sunlight
or heat. Brown topaz can be bleached by sunlight and yellow
topaz turns pink or purple-red when exposed to moderate
heat. A Parisian jeweller discovered this latter phenomenon
in 1750 and used it to create the first artificially enhanced
pink topaz. Today nearly all the pink topaz being sold
in jewellery has been heat treated to turn it pink. |
 |
Topaz
is a silicate and often occurs in deposits of granite
associated with other minerals. It is also commonly found
as rounded pellets in alluvial deposits. The most notable
occurrences are in Brazil, Pakistan, Nigeria, Australia,
Tasmania, Sri Lanka. Russia, USA, Mexico, China and Burma.
A
chain like structure of connected irregular octahedrons
controls the structure of topaz. These octahedrons have
one aluminium atom in the middle surrounded by four oxygen
atoms. Above and below the aluminium are the hydroxide
or fluoride ions. The chains of octahedrons are held together
by individual silicate tetrahedrons but it is the octahedrons
that give topaz its crystalline shape. Why this information
is important is that inspite of being one of the hardest
minerals and the hardest silicate mineral, toting up 8
on Moh's scale, it has a perfect cleavage that U perpendicular
to the chains and ix caused by the planes that break the
weaker aluminium - oxygen, aluminium - hydroxide and aluminium
- fluoride bonds. None of the stronger silicon - oxygen
bonds cross these planes. |
|
Topaz
crystals can consequently reach huge sizes and weigh as
much as several hundred pounds. Topaz's crystal habits
alst makes it a very attractive mineral specimen due to
its high lustre, nice colours and well formec multifaceted
crystals. The largev uncut stone, a specimen found in
Brazil weighing almost 600 pounds is on display at the
American Museum of Natural History in New York City. Its
crystalline formation also allows the gem to hold an electric
charge for up to thirty hours. In fact, you can charge
topaz by the simple act of rubbing it between your fingers.
Some Brazilian stones get a charge just by holding the
ends between your fingers. Heating the gem and allowing
it to cool slowly builds an electrified charge greater
than any achieved with other stones and the topaz will
retain the electrical energy for more than a day after
cooling down.
|
|
Topaz
from most sources is reasonably clean. Thus eye clean
stones are both desirable and available. The exceptions
are pink and red topaz, which are usually found only in
small sizes and are often included. In fact, fine pinks
and reds of more than five carat size are very rare and
orange stones in sizes of more than twenty carats are
also uncommon.
Topaz
contains upto 20% water and fluorine and the relative
proportions of these impurities are what cause the colour
in the natural stones. Crystals with more water are yellow
to brown, while those with more fluorine are typically
blue to colourless. Except in some red and pink stones
where trace quantities of Chromium act as a chromophore,
the colour in topaz is due to colour centres which is
why they are good candidates for colour change through
irradiation and heating. |
|
|
| |
1. and 2. Mystic fire topaz
5. Smoky topaz
6. Padparadscha coloured diffused topaz
7.Natural pink topaz |
| |
Topaz
as mentioned earlier, is rarely sold unenhanced and the most
common enhancement technique used is irradiation to turn the
stones blue. Sky, Swiss and London Blue are all shades of blue
achieved by irradiation. The most widely used techniques are
explained below. The first method is the Gamma Ray Treatment
where exposure to a gamma ray source (usually cobalt 60 is used)
will produce both blue and yellow colour centres resulting in
a brownish or brownish - green colour in most of the stones.
Subsequent heating removes the less stable yellow colour centres
without affecting the blue. Such treatment, though it was once
very commonly used is no longer popular today as it is not capable
of producing the darker, purer blues that are in the
highest demand and that can be created by
other techniques The light blue colour produced by gamma ray
treatment has been given the name of Cobalt Blue. Darker blue
stones that have undergone this treatment are often greyish
or Steely Blue. Gamma ray treatment is more often used today
as a pretreating or screening process to reveal stones that
are more likely to be susceptible to treatment with the higher
energy techniques and such stones take on a bluish tint during
this initial process.
|
| |

1. Bi Colour topaz is one of the diffusion treated topaz created
by US firm Leslie & Co. |
| |
Linear
Accelerators or Linac treatment exposes topaz to high-energy
electron beams. The process generates considerable heat and
the topaz has to be water cooled to prevent thermal shock and
spontaneous destruction of their colour centres. This process
too has to be followed by heat treatment in order to destroy
the unwanted and less stable yellow colour centres. The result
of this treatment is a darker blue colour, with little to no
grey and is popularly known as Sky Blue. These stones are radioactive
for a while after the treatment and must be allow to sit for
a couple of weeks to allow the residual radiation to decline
to safe levels.
Then
there are the nuclear reactors where topa/ is exposed to fast
neutrons, producing blue colour. No subsequent heating is required
here. The colour produced is a medium to dark greyish blue that
is called steely or inky blue and goes under the trade name
of London Blue. Heat treatment in fact, can be used to lighten
the inkiness. Material treated in the nuclear reactor is likely
to be quite radioactive and may require more than several months
of storage before the radioactivity decays to safe levels. |
| |
 |
| |
There
are also combination treatments that use a sequence of nuclear
reactor, linear accelerator and heating to produce dark blue
colours without the inkiness of London Blue. These goods go
under a variety of names like Electra Blue, Super Blue, New
Blue, Swiss Blue, Max Blue. American Blue. California Blue and
Super Sky Blue.
In
recent years, there has been the development of one more treatment
process for topaz that has yielded beautiful results. This is
the diffusion process, which is normally used for corundum.
Diffusion is a process whereby a natural colouring agent such
as iron oxide for example, is driven into a faceted natural
colourless gemstone in a way that makes the colour a permanent
part of the crystal lattice. This is done by heating the gem
with the colouring agent to a very high temperature in
a pressurised furnace. Atoms ot the colouring
agent enter the gem and replace certain atoms just like they
do in nature.
|
| |
Essentially,
the process is just a modified version of the one used
for sapphire. However, the downside is that not all topaz
can he successfully treated this way. As the gems are
heated to around 850"C-950"C, some of the stones
that have gases trapped inside tend to break during the
heating process and therefore only the most durable gems
can be used. Ideally, it is the Brazilian goods that are
best suited for diffusion treatment. Also despite its
hardness that measures 8 on the Moh's scale, topaz is
actually quite brittle because of its crystal structure
i as we described earlier. Therefore breakages during
treatment account for a significant percentage of the
goods.
The
reason why only faceted gems are used is that the colour
is only skin deep and polishing or even a deep scratch
will result in exposing the underlying colourless stone.
However, the colour itself is permanent and will not fade
regardless of the amount of exposure that it gets to sunlight.
As only the most durable pieces that make it through the
diffusion treatment, diffused topaz can easily be cleaned
in a steam or ultrasonic cleaner without fear of cracking
or breaking. It is heat resistant to re-tipping on the
gemstone; it can withstand pickle (for resizing) and plating.
Additionally most manufacturers will accept the goods
back for retreatment if there is colour loss due to scratching
or breakage so that is a benefit.
|
|
 |
The
main advantage of the diffusion process is that
unlike the irradiation treatment that only produces
blue, diffusion can produce a whole spectrum of
colour that ranges from many shades of blue, blue-green,
green, yellow, orange and even the ever fascinating
padparadscha colour. Generally though, the range
is widest in the green and blue-green colour range.
The other advantage is that since it does not
undergo any form of irradiation, it is more trader's
point of view does not block up large quantities
of goods for long periods of time for the cool
down period while the residual radioactivity decays.
Again irradiation is done to huge quantities of
goods in order to be cost effective while diffusion
can also be done on relatively smaller lots so
that even small traders can effectively place
their own orders without having to source through
a |
 |
|
manufacturer. Since
this is a new process though, there are only a handful
of companies that are doing diffusion treatment
in the USA and India. The most famous of these are
Leslie & Co., in the United States and Pink
City Gem Technologies in India who have their own
brand called Spice Topaz besides providing the service
to other manufacturer-traders as well. |
Thus
we see that topaz has gone full circle in the last century
from being known as a primarily yellow gem to becoming
famous as a mainly blue coloured stone and now, in the
future if diffused topaz becomes a great hit, it will
be known as a multi coloured gem because the diffusion
technology is still being fine tuned and every few months,
new colours are being produced. It will not be very long
then when a generation that once regarded blue and topaz
to be two halves of the same word, will come to appreciate
this gem for its chameleon like ability to take on every
colour in nature and some besides that as well.
|
|
| |
|
|







