Results
Long Term UV Exposure
Of particular interest to
this study, however, is the reaction of the stones after
long-term exposure to intense UV light. The Beryllium-Treated
samples from Madagascar reacted differently to all other
samples, excluding unheated ones (See Table A1). Significant
changes in the color were seen in samples heated with Beryllium,
and a shift towards more yellow or orange was observed (as
shown in the Table A1). A color change from near colorless
to yellow was also observed in untreated sapphires of Sri
Lankan origin (Table A1). This unheated group of sapphires
is known to contain specific color centers (Lit. 16). In
Beryllium-Treated gemstones with an orange body color before
the UV experiments, the color shift is more difficult to
see. The color change towards more yellow or orange could
be reversed when exposed to a gas flame for a short time,
and within two days when exposed to a 100 watt halogen lamp
at slightly elevated temperatures. A set of white sapphires,
which did not change color during the Berylllium-treatment
process developed a thin layer of yellow color during the
long term UV exposure (shown in Fig. A4). This observation
was also made on Beryllium-Treated blue sapphires (Fig.
A4) which have been exposed to UV. The blue samples developed
orange edges, while the body color of the sapphires remained
unchanged. The color induced by UV treatment could be reversed
by short application of heat to all the samples, beside
one exception.
Scanning Electron
Microscope Analysis (SEM)
Sampling of colored sapphires
heated by the new treatment in Chantaburi included the study
of faceted Beryllium-Treated gemstones (Materials group
f.). These gemstones were already faceted in briolette and
princess-cut style sapphires prior to heat treatment. By
sorting the lots after heat treatment, a set of non-sapphire
materials were detected (Fig. A8). The colored samples showed
a thin film of interference color at the surface, and were
indented by craters due to contact with other minerals in
the same heating process. These craters were also found
indented on the surface of the sapphires (Fig. A13). The
materials were identified as consistent with glass-aggregates,
zircon and chrysoberyl (Table A2).
Furthermore, clusters of sapphires,
sintered together by a whitish matrix, were detected. Only
an extremely small portion of the gemstones showed this
phenomena and it was clear that these were accidental circumstances.
In order to analyze the surfaces
of these materials, and to search for potential trace elements
used in the process, the materials were studied with a Scanning
Electron Microscope (Philips XL 30 ESEM) in February 2002
at the University of Basel's Central Laboratory for Microscopy
(ZMB) by Chief Technician M. Duggelin and D. Mathys. Five
samples were selected (including minerals with an interference
film (zircon and chrysoberyl), and sapphire clusters |
|
Results
SEM
Analyses of
zircon and chrysoberyl did not reveal any further
information on chemicals present, other than expected
from their chemical compositions and attention was
placed on the sapphire clusters and the white matrix
around the sapphire materials. Cracks and intended
craters were investigated, and a series of newly
formed crystallizes were detected in these cracks
(Fig. A15-A17), mostly composed of Zr-oxide, plus
additional element Silicon (Si), Aluminium (Al)
, Magnesium (Mg), Calcium (Ca) and Fluorine (F).
Beryllium can not be measured with SEM, and no indications
for Chromium (Cr), Titanium (Ti), or Iron (Fe),
were found on the surfaces of the enhanced gemstones.
Most of the detected elements can be explained as
originating from decomposed minerals present in
the runs (Silicon (Si) and Zirconium (Zr) from zircon,
Aluminium (Al) from corundum, or chrysoberyl), yet
the source of Fluorine (F) is unclear. Melting on
the surface of these minerals is very visible, as
different craters are present on the surface of
the former faceted materials (Fig. A8) and also
by the craters produced at the surface of the sapphires
(Fig. A13, A14). They were formed when they came
into contact with other chemical compositions present
in other minerals - of the same shape, cutting style
and size - in the heating run. Chrysoberyl is a
potential source for Beryllium. Its role in the
heat treatment process has been confimed (See later
publications Lit. 23-33).
|
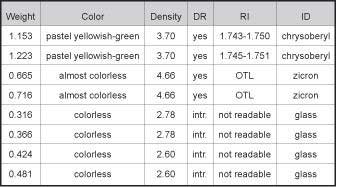 |
Table
A2 :Selected representative ID Data on
the identified minerals accompanying the sapphires
heat-treated in the presence of Beryllium. Samples
cut in half and polished. |
|
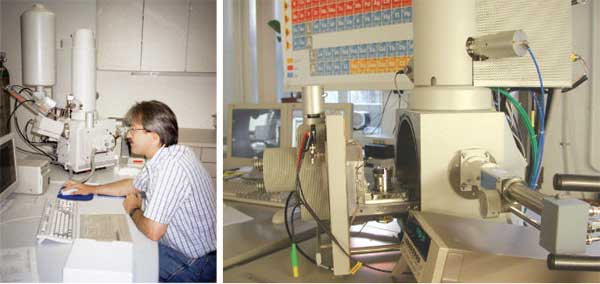 |
Fig. A7
An example of a SEM-EDS recently used for research
is shown from the University of Fribourg, Earth Science
Department, Switzerland. On the left: Prof.B. Grobety
operating the SEM (FEI XL 30 Sirion FEG). To the right
details of the SEM: An opened sample chambre, surrounded
by a variety of detectors, such as Secondary Electron
Detector (SE), Backscattered Electron Detecor (BSE)
and an X-Ray Detector (EDS) with a Polymer Window
(S-UTW), for microchemical analysis. |
 |
Fig.
A8 A set of non-sapphire materials sorted
after heat treatment experiments. In December 2001,
heat treatment was carried out with the new method
in Chantaburi (Thailand) on a lot of briolette sapphires
and then inspected by GRS at the source. First row:
Method no-sapphire material transformed to whitish
glass-aggregates. Second row: Zircon miterals (radioactive).
Third row: Chrysoberyl minerals. (Note: Creters on
the surface of the zircon and chrysoberyl crystals
due to contact with other minerals in the heat treament
process and partial melting of the surface, See arrows).
Overgrowing layers of thin films with interference
color were found on zircon and Chrysoberyl samples,
indicating some chemical reactions on their surfaces.
Samples collection GRS. |
|