|
Prestigious Innovations Prize awarded at Inhorgenta
Europe
Each
year at Inhorgenta Europe, the Innovations Prize is awarded for outstanding.,
future-oriented jewellery design. The winners of the prize awarded
at the recent Inhorgenta Europe 2004 were Claudia Hoppe from Germany,
Norika Matsumoto from |
|
| |
England,
Yvonne Raab from Germany and Karin Wagner from Switzerland.
In the five years since the award was launched, it has become
one of the most prestiqious prizes in the design sector. Inhorgenta
Europe, with 300 exhibitors alone in the designer jewellery
section, has risen to become the biggest platform for designer
jewellery worldwide. |
|
 |
|
|
| |
For
the Inhorgenta Europe 2004 Innovations Prize, 151 designers
submitted nearly 450 pieces. The creations came from 10
countries -- Belgium, Germany, France, Great Britain, Italy,
Japan, Poland, Switzerland, Spain and Taiwan. From these
the four winners were chosen by an international jury of
recognised experts, comprising Angela Oelckers, editor-in-chief
of AMICA (Hamburg), Monica Gaspar Mallol, curator of contemporary
jewellery at the Museum of Applied Arts (Barcelona), Prof.
Susan Cross, lecturer at the Edinburgh College of Art, Jytte
Kloeve, a designer from Lyng-by (Denmark) and Isabella Hund,
a gallery owner from Munish.
The
prize-winning pieces were displayed in a special exhibition
at Innorgenta Europe, on 'Desingers' Avenue ' where rhe
awards ceremony also took place. the winning designers gave
some insight into their designs and influences.
"My jewellery is an invitation
to you to decorate yourself, or people you like, in a way
that pleases you. Some of my pieces - - with their colourfulness
and provocative combination of stainless steel and plastic
- - are just asking the wearer to expiriment with them.
Individual components can be switched around, so that the
wearer can put together their own very individual items
of jewellery. Others are more restrained, yet their classic
forms and traditional materials contain a secret that, depending
on taste, can be found out by thinkingabout it or trying
it out. Enjoy, "said, Claudia Hoppe, one of the four
winning designers of the Innovations Prize. |
|
 |
|
|
| |
"My
collection focuses on the transformation of shapes. Shapes communicate
different meanings depending on how they are manipulated by altering
their scale, volume or construction . I intend to investigate ther
design potential of these alternatives and develop them into jewellery
pieces. The basic shape I shall use is anonymous, abstract and simple
yet gives a familiar impression to audience. In this collection,
I develop it in two dimentions, using line and volume. As for materials,
silver is employed as the main one. The neutrality of its colour
and quality is a very interesting feature for me. These aspects
can encourage me to reveal clear impressions from each form. In
order to emphasise them I focus on three colours, silver, black
and gold, " explained award winner, Noriko Matsumoto. |
|
| |
|
|
| |
"The
inspiration comes from the mountains, my home,
from memories of childwood, from the many beautiful
colours of nature. I love the combination of
romance, tradition and contemporary design,
mixed with a pinch of humour. To sum up, my
jewellery creations are characterised by three
criteria: romatic playfulness combined with
'coolness', a mixture of tradition, history
and fashion design, and a combination of craft,
art and design," said award winner, Karin
Wagner. |
|
www.inhorgenta.com Images
copyright: Messe Munchen GmbH/Photos: Loske
(le)
|
|
Large
diamonds made from gas are the hardest yet
Producing
a material that is harder than natural diamond has
been a goal of materials science for decades. Now
a group headed by scientists at the Carnegic Institution's
Geophysical Laboratory in Washington, D.C., has produced
gem-sized diamonds that are harder than any other
crystals . Futher, the reserchers grew these diamonds
directly from a gas mixture at a rate that is up to
100 times faster than other methods used to date.
"These
are real diamonds made of carbon and identical in
structure to those formed in nature and by high pressure
and temperature methods, " siad Chih-shiue Yan,
lead author of the study published in the February
20, online Physica Status Solidi. "We believe
these results are major breakthroughs in our field.
Not only were the diamonds so hard that they broke
the measuring equipment, we were able to grow gem-sized
crystals in about a day."
The reserches
grew the crystals using a special high-growth rate
chemical vapor deposition (CVD) process that they
developed. They then subjected the crystals to high-pressure,
high-temperature treatment to further harden the material.
In the CVD process, hydrogen gas and methane are bombarded
with charged particles, or plasma, in a chamber. The
plasma prompts a complex chemical reaction that results
in a "carbon rain" that falls on a seed
crystal in the chamber. Once on the seed, the carbon
atoms arrange themselves in the same crystalline structure
as the seed. In thiscase, the seed was a type 1b synthelic
diamond plate. They have grown single crystals of
diamonds up to 10 millimeters across and up to 4.5
millimeters in thickness by this method.
|
|
|
|
| |
|
|
| |
The
crystals produced by CVD are very tough, "We noticed this
when we tried to polish them into brilliantcuts, "said Yan.
"They were much harder to polish than conventional diamond
crystals produced at high pressure and high temperature."
The researchers then subjected the tough CVD crystals to high-temperature
and high-pressures between 50,000 and 70,000 times atmospheric
pressure (5-7 GPa) for ten minutes. This final process resulted
in the ultrahard material, which was at least 50% harder than
the conventional diamonds as shown by direct measurements carried
out in collaboration with scientists at Los Alamos National Laboratory.
"Making
diamonds has not been the primary goal of our research,"remarked
Russell Hemley of Carnegic. "our group is interested in the
behavior of materials at extreme pressures and temperatures. We
need large, perfect diamond crystals to create new classes of
high-pressure devices for our research and decided to explore
whether we could make these crystals by CVD processes. We found
that we could, and at a very high growth rate. This has opened
up an entirely new way of producing diamond crystals for a variety
of applications, such as the next generation diamonds-based electronic
devices and cutting tools. Our new finding that the diamonds can
be supertough and/or superhard was a surprise and will greatly
benefit many of these applications."
This
research was supported by the National Science Foundation, the
U.S. Depertment of Energy, the National Nuclear Security Agency,
through the Carnegie/DOE Alliances Center, CDAC, and the W.M.
Keck Foundation . It was conducted in collaboration with researchers
at the Phoenix Crystal Corporation and Los Alamos National Laboratory.
The Carnegie Institution of Washington (www.CarnegieInstitution.org)
has been a pioneering force in basic scientific research since
1902. It is a private, nonprofit originization with six research
depertments in the U.S. : Plant Biology and Global Ecology in
Standard, CA; The Observatories in Pasadena, CA, and Chile; Embryology,
in Baltimore, MD; and the Department of Terrestrial Magnetism
and the Geophysical Laboratory in Washington, DC.
http://carnegienstitution.org
|
|
|
Lead-glass Impregnated
Ruby GAAJ Lab. Alert - - Research Laboratory, Gemmological Association
of All Japan
We encountered
a ruby which showed flash effect from fissures and fractures. Our
analysis revealed that the stone was impregnated with lead-bases
glass. This report describes details of the material. |
|
| |
Treating
diamonds by impregnating cleavages or fractures with highly
refractive glass to improve the clarity (transparency) has
been commonly known. This type of treatment appeared on the
market around 1987 and has been called glass "filling."
It is also called "Koss" treatment or "Yefuda"
treatment, named after the treaters. Cleavages and fractures
in these treated diamonds show unique rainbow-coloured light
called the flash effect. This optical effect is caused by
the difference in dispersion between diamond and the impregnating
material, but refractive indices of the two materials should
be almost overlapping. In other words, a host gemstone and
an impregnating material should have very close refractive
indices and different dispersions to show the flash effect. |
|
|
Photo
1:A 13.220 ct ruby impregnated with lead-based glass |
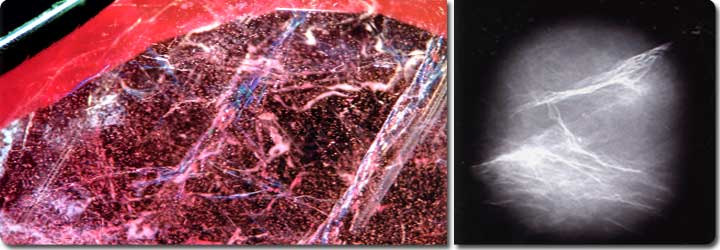
Photo-2: Flash effect is seen from fissures and fractures.
Photo-3: A radiograph. White streaks in high contrast correspond
to the distribution of fractures. |
This
flash effect recently started being seen in some faceted ruby.
Shown in the Photo-1 is a 13.220 ct ruby, whose internal features,
spectral analysis and other tests indicated that the stone
has been heated and may have its origin in regional metamorphic
rock such as found in African corundum fields. This ruby was
faceted but many cracks reaching to the surface were observed
inside the stone. From these cracks, an odd blue to purple
light effect (flash effect), which was different from ordinal
interference of thin film, was observed (Photo-2). Residues
from heating processes may often be seen in fractures and
cavities in ruby, but they never produce a lower refractive
indices than ruby; thus, they never produce a flash effect.
The flash effect seen in ruby indicates that the stone is
impregnated with substances whose refractive indices overlap
that of ruby. To identify the substance and to confirm the
degree of the impregnation, we performed an X-ray test and
X-ray fluorescence analysis. The radiograph is shown in the
Photo-3. Many white streaks in high constrast correspond to
distributed fractures developed in ruby. The uniform white
part in the radiograph indicates existence of elements which
have larger atomic mass than the host ruby (Al203).
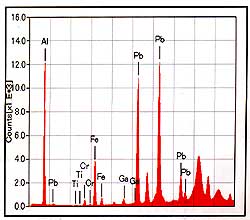
Figure-1
shows the results of measurements by X-ray fluorescence analysis
of the fractures reaching to the surface. In the results,
detection of Pb(lead) stands out beside the main chemical
element of Ruby, AI, and common trace elements. The existence
of light elements such as boron cannot be denied at the present
stage, because the X-ray fluorescence analysis cannot detect
those light chemical elements. |
From
the observation and analysis described above, the newly appearing
ruby showing the flash effect must be highly fissured or fractured
ruby from Africa or other sources, which have been impregnated
with lead-based glass having a similar refractive index, for
the purpose of clarity improvement.
GAAJ
REsearch Laboratory. Copyright 2004 Zenhokyo Co.,Ltd. All
rights reserved. Reproduced by permission.
www.gaaj-zenhokyo.co.jp |
|
 |
| |
|
Figure-1: Results of X-ray
fluorescence analysis on fractures.
|
The
Israel diamond Musuem opens rare gemstones & minerals
exhibition
The Harry Oppenheimer
Diamind Musuem in Ramat Gan, has opened a unigue exhibition
of gemstones and minerals, |
|
|
containing
500 rate items, gathered from 55 countries around the
world. The private collection of Ben-Zion and Sarah
Harel, accumulated over a period of 30 years, this unique
treasure will form part of the permanent collection
of the museum. Among
the unique pieces on display are a prehistoric insect,
resembling a mosquito, trapped in amber over 20 million
years ago. Also featured is two billion year-old crystal
from Nambia, containing a droplet of water, which moves
freely within the stone. Another highlight is the largest
ruby in the world - - weighing 1908 grams, originating
from Kenya.
Other
exhibits include, a 25 million year-old red emerald
embedded in stone. "Emerald Crystal Twins"
from Northern Afghanistan - a perfectly-formed crystal
that sprouted a second perfect crystal, and a fossillized
tree trunk which grew calcite crystals - an exceedingly
rare combination in nature weighing 5112 grams. |
Emerald
crystal |
|
|
|
Ben-Zion
Harel is an international gemstone dealer, who founded and
headed for more than a decade the israel Emerald Cutters Association.
He was also a founding member of the International Colored
Gemstone Association. Mr Harel, whose family has been actively
trading gemstones between Europe and the Orient since the
19th century, has a deep knowledge and affinity for minerals
and semi-precious stones. he created the collection from pieces
acquired on is many trips around the world.
The
Harry Oppenheimer Diamond Museum, a subsidiary of the Israel
Diamond Institute is located in the Maccabi Building within
the Diamond Exchange complex. It features unique permanent
and changing exhibitions presenting the magical world of diamond,
gemstones and gem-studded jewelry.
|
|
Left
to right : 1) Kunzite crystal 2) Ruby crystal 3) Red beryl
crystal
www.diamonds.org.il/Museum.html
|
|
|
|

